■ Example of research topics ■
[ Solar ]
1. 4 junction by wafer bonding /
2. High-speed MOVPE /
3. EL and PL characterization /
4. Thin-film multi-junction (design and light trapping) /
5. 1.15 eV middle cell with MQWs /
6. Theoretical modeling of MQWs (quasi bulk approach and cell design) /
7. WoW /
8. ELO /
9. Dilute nitride MQW
[ Growth ]
1. III-V on Si photo detector /
2. III-V on Si solar cell
[ LED ]
1. Chip-white LED
[ Solar Fuel ]
1. CPV + water electrolysis /
2. CO2 reduction /
3. Semiconductor/electrolyte interface /
4. Polarization-controlled nitride photocathode /
5. Design of energy management system using hydrogen-based power storage
[ Quantum Modeling ]
1. Quantum modeling of insulators
Polarization-controlled nitride photocathode
H. Maruyama, K. Fujii (RIKEN) and M. Sugiyama
GaN is known as a material as a semiconductor photo-electrode for water-splitting. In water splitting operation, n-type semiconductor functions as photoanode, while p-type semiconductor as photocathode. As for the stability of photo-electrode, photocathode (an electron injector) is tolerant against corrosion, which proceeds via the accumulation of holes on the surface. However, photocurrent of p-type GaN has been much less than that of n-type GaN, as a result of a poor crystal quality of p-type GaN. Our group has invented a polarization-engineered structure with thin AlN layer embedded in GaN, which functions as a photocathode with larger photocurrent than the conventional photocathode using p-GaN. The photocathode operation makes the polarization-engineered electrode more tolerant against surface corrosion than n-type GaN photoanodes. This structure is thought to promote water splitting by sunlight without external bias appliation. Especially, simulated turn-on potential of photocurrent is much higher than oxygen generation potential E°(O2/H2O), which is essential for unbiased water splitting. However, results of experiments showed that there were voltage loss larger than 1 V, and the turn-on potential was very close to E°(O2/H2O) and photocurrent at E°(O2/H2O) was too small. So, further optimizations of (In)GaN/AlN/GaN structure, such as depositing Pt co-catalyst on surface, introducing dual tunneling junctions and growth of the structure with low threading dislocation density are needed to enable unbiased water splitting using the polarization engineered photocathode.
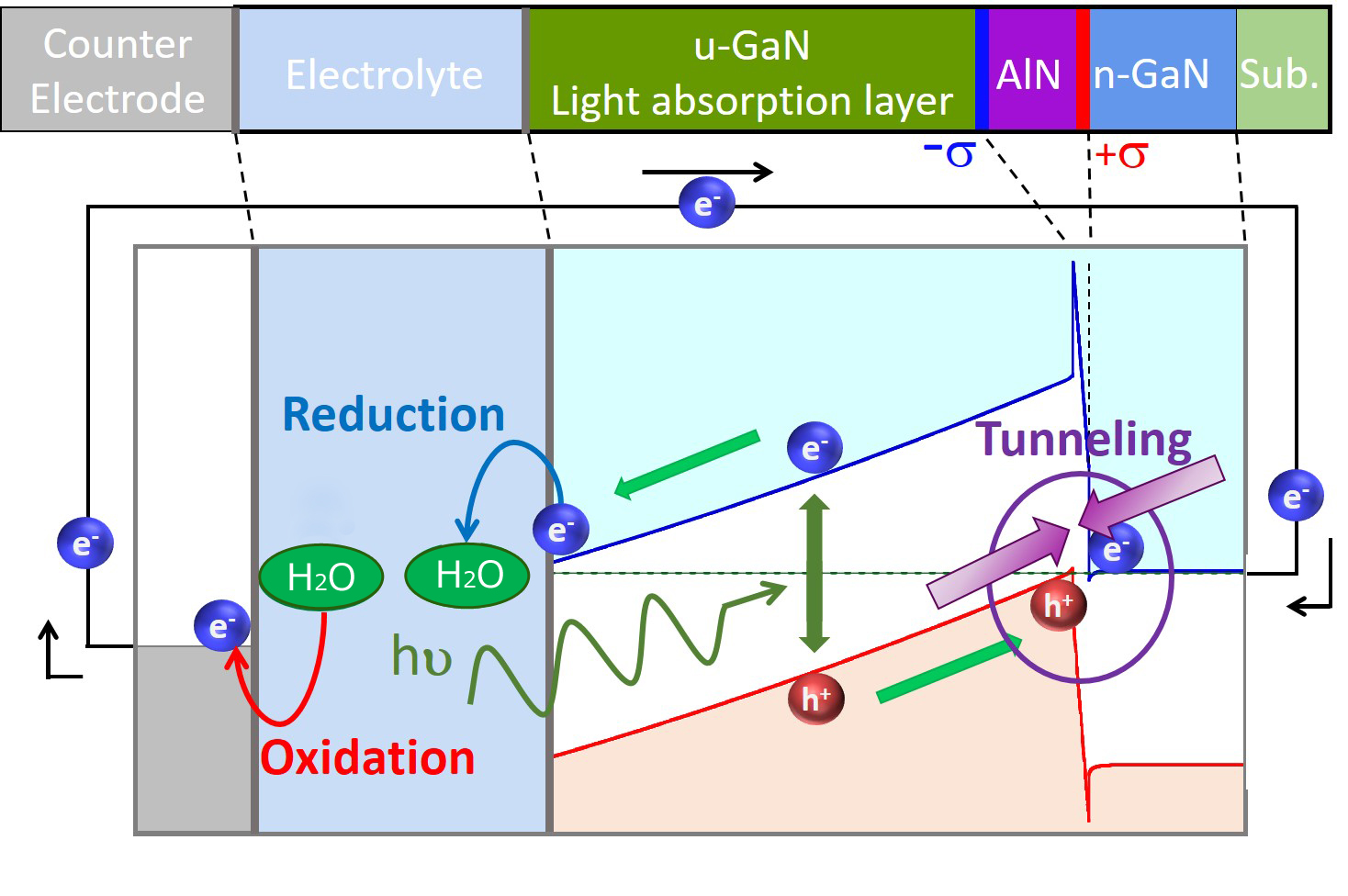
Fig. 1 The layer structure and operation principle of a polarization-engineered GaN photocathode
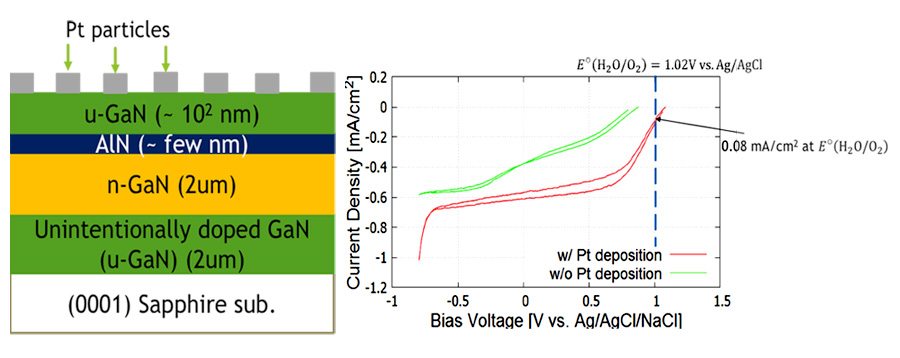
Fig. 2 An example of improved I-V characteristics for the polarization-engineered photocathode by the surface modification of platinum co-catalysts