■ Example of research topics ■
[ Solar ]
1. 4 junction by wafer bonding /
2. High-speed MOVPE /
3. EL and PL characterization /
4. Thin-film multi-junction (design and light trapping) /
5. 1.15 eV middle cell with MQWs /
6. Theoretical modeling of MQWs (quasi bulk approach and cell design) /
7. WoW /
8. ELO /
9. Dilute nitride MQW
[ Growth ]
1. III-V on Si photo detector /
2. III-V on Si solar cell
[ LED ]
1. Chip-white LED
[ Solar Fuel ]
1. CPV + water electrolysis /
2. CO2 reduction /
3. Semiconductor/electrolyte interface /
4. Polarization-controlled nitride photocathode /
5. Design of energy management system using hydrogen-based power storage
[ Quantum Modeling ]
1. Quantum modeling of insulators
Electrochemical carbon dioxide (CO2) reduction for solar fuel production
Fahd S. Khan, K. Fujii (RIKEN), Y. Nakano and M. Sugiyama
Carbon dioxide (CO2) is the primary greenhouse gas emitted through human activities and it has begun to alter the carbon cycle of our planet. If nothing is done to reduce CO2 emissions significantly over the coming decades, the resulting global warming and the consequential impact can put the entire planet at risk of destruction.
Moreover, it is possible to reduce CO2 with electricity, to synthesize sustainable fuels. If the electricity is obtained via photovoltaic power generation or other renewable electricity sources, the synthesized fuel can be regarded as package of solar energy, in other words, “solar fuel.” This approach can provide a feasible mechanism to use CO2 as an energy carrier in the form of liquid fuels, by transportation from areas of abundant renewable energy resource to regions of high energy consumption. This mechanism will allow us to make a transition of our energy source from conventional fossil fuel to more sustainable solar-based source.
Previously, pioneering researchers have attempted electrochemical CO2 reduction using standard setup with an electrolyte saturated with CO2. A variety of metal electrodes have been examined over the years for reduction of CO2 into viable products however, in general, these systems have required relatively large overpotentials, and have exhibited limited operational lifetimes. Recently focus has shifted away from metals and on to finding new materials, which could especially allow electron-intensive reduction of CO2. The CO2 reduction research in our lab focuses on structurally defective materials such as chalchogenides, conductive glasses and graphene-oxide for possible reduction of CO2 into useful products.
More fundamental improvement in the selectivity of hydrocarbon products over hydrogen, which has been a severe bottleneck of electrochemical CO2 reduction especially under high current density, will necessitate a reactor based on the concept of fuel cells. By suppressing the supply of proton via membrane transport, we can alter the reactant concentrations around the cathode surface in favor of hydrocarbon production. By developing a cathode catalyst which can selectively produce hydrocarbons under such an optimized reactant environment, practical electrochemical reactor for CO2 reduction will be realized.
Studies to show the practical feasibility of CO2 reduction by developing PV coupled electrochemical reactors are also underway. Our lab works in close collaboration with groups pursuing similar research at RIKEN (Japan), Princeton University (USA) and Saint-Petersburg State University (Russia).
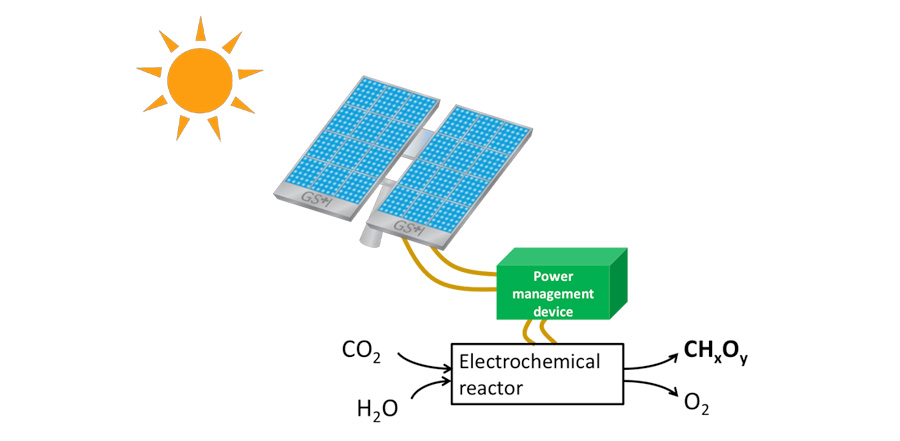
Fig. 1 A schematic of an apparatus for generating solar fuel by the reduction of CO2 powered by sunlight
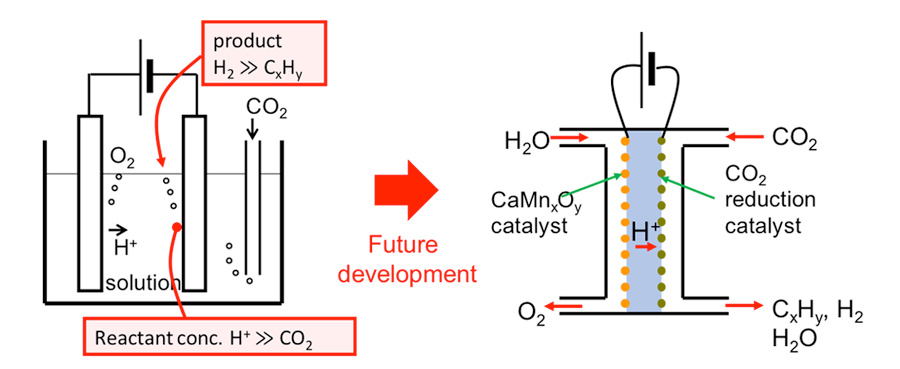
Fig. 2 A proposal of fuel-cell-type electrochemical cell to reduce CO2 effectively into hydrocarbons by the control of reactant concentrations.