■ Example of research topics ■
[ Solar ]
1. 4 junction by wafer bonding /
2. High-speed MOVPE /
3. EL and PL characterization /
4. Thin-film multi-junction (design and light trapping) /
5. 1.15 eV middle cell with MQWs /
6. Theoretical modeling of MQWs (quasi bulk approach and cell design) /
7. WoW /
8. ELO /
9. Dilute nitride MQW
[ Growth ]
1. III-V on Si photo detector /
2. III-V on Si solar cell
[ LED ]
1. Chip-white LED
[ Solar Fuel ]
1. CPV + water electrolysis /
2. CO2 reduction /
3. Semiconductor/electrolyte interface /
4. Polarization-controlled nitride photocathode /
5. Design of energy management system using hydrogen-based power storage
[ Quantum Modeling ]
1. Quantum modeling of insulators
Investigation of photoelectrochemical reaction by extended semiconductor physics
Yuki Imazeki, Katsushi Fujii (RIKEN), Yoshiaki Nakano and Masakazu Sugiyama
Solar energy cannot be supplied stably. To solve this problem, energy storage through the conversion of solar energy to chemical energy is expected. Hydrogen is one of the attractive chemical substance for energy storage for its high energy density. The highest energy conversion efficiency from solar to hydrogen was achieved by connecting photovoltaic modules with water electrolyzers. But, its cost is still expensive. Therefore, the other methods, photocatalysts and photoelectrodes (semiconductors in electrolyte) are expected for low cost energy hydrogen production via solar-powered water splitting. However, the energy conversion efficiency and durability are still low. For higher efficiency and durability, surface of semiconductor have been modified by co-catalysts and protection layers. Co-catalysts decrease activation energy for water splitting reaction and enhances the production rate of hydrogen. Protection layers prevents dissolution of semiconductor into an electrolyte by avoiding the direct contact between an electrolyte and a semiconductor. To optimize material and structure of such surface modifiers, understanding carrier transport through the interface between semiconductor and molecules in electrolyte is required. To understand mechanism of the carrier transport, band alignment, which is well-known in semiconductor physics, needs to be clarified. The band alignment is influenced by adsorbed ion and surface states of semiconductor, such as defects, oxidized layers and the existence of additional surface modifiers. Their effect on the band alignment is being evaluated to clarify the carrier transport mechanism.
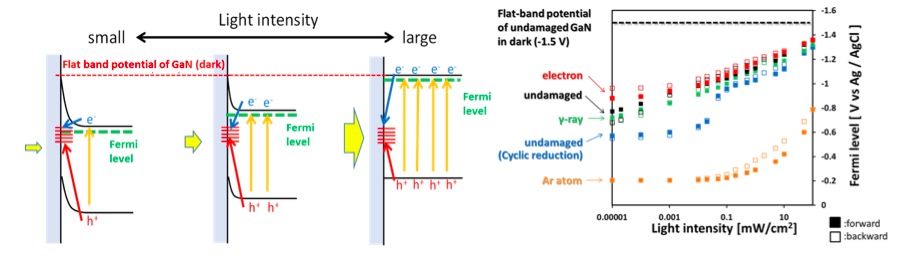
Fig. Band alignment evaluation at interface between a semiconductor and an electrolyte